 The brain is composed of thousands of distinct types of neurons and glia that enable it to perform such complex tasks. During development, these specialized cells must be generated in correct numbers, migrate to appropriate positions, and wire together in a very precise way. Subtle disruptions during any one of these stages can drastically alter the way the brain forms and functions, resulting in a neurodevelopmental or neuropsychiatric disorder, such as autism spectrum disorder (ASD), intellectual disability (ID), or schizophrenia. Intriguingly, growing evidence indicates that DNA sequence alone cannot fully explain susceptibility to such cognitive disruptions, which are likely caused by a complex interplay between the genome, epigenome, and environment. The goal of our lab is to understand how these factors intersect to orchestrate development of the brain.
The brain is composed of thousands of distinct types of neurons and glia that enable it to perform such complex tasks. During development, these specialized cells must be generated in correct numbers, migrate to appropriate positions, and wire together in a very precise way. Subtle disruptions during any one of these stages can drastically alter the way the brain forms and functions, resulting in a neurodevelopmental or neuropsychiatric disorder, such as autism spectrum disorder (ASD), intellectual disability (ID), or schizophrenia. Intriguingly, growing evidence indicates that DNA sequence alone cannot fully explain susceptibility to such cognitive disruptions, which are likely caused by a complex interplay between the genome, epigenome, and environment. The goal of our lab is to understand how these factors intersect to orchestrate development of the brain.
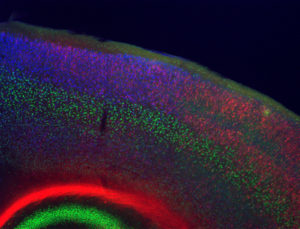 The molecular and cellular mechanisms that mediate interactions between genetic and environmental factors are poorly understood. To tackle this complex biology, we study how environmental factors and nutrition (e.g. vitamin D, folic acid, high-fat diet) modify neuronal development and function in both normal mice and mice with genetic mutations that model neuronal disruptions in human neurological disorders. We are particularly interested in epigenetic factors since the epigenome sits at the interface between genes and the environment, and disruptions in epigenetic regulation lead to numerous disruptions in neuronal development and function. Two of our current projects focus on genetic mutations in epigenetic regulators – Mecp2, mutations in which lead to the severe neurological disorder Rett syndrome, and Cited2, mutations in which lead to neural tube closure defects and disrupted neocortical development.
The molecular and cellular mechanisms that mediate interactions between genetic and environmental factors are poorly understood. To tackle this complex biology, we study how environmental factors and nutrition (e.g. vitamin D, folic acid, high-fat diet) modify neuronal development and function in both normal mice and mice with genetic mutations that model neuronal disruptions in human neurological disorders. We are particularly interested in epigenetic factors since the epigenome sits at the interface between genes and the environment, and disruptions in epigenetic regulation lead to numerous disruptions in neuronal development and function. Two of our current projects focus on genetic mutations in epigenetic regulators – Mecp2, mutations in which lead to the severe neurological disorder Rett syndrome, and Cited2, mutations in which lead to neural tube closure defects and disrupted neocortical development.
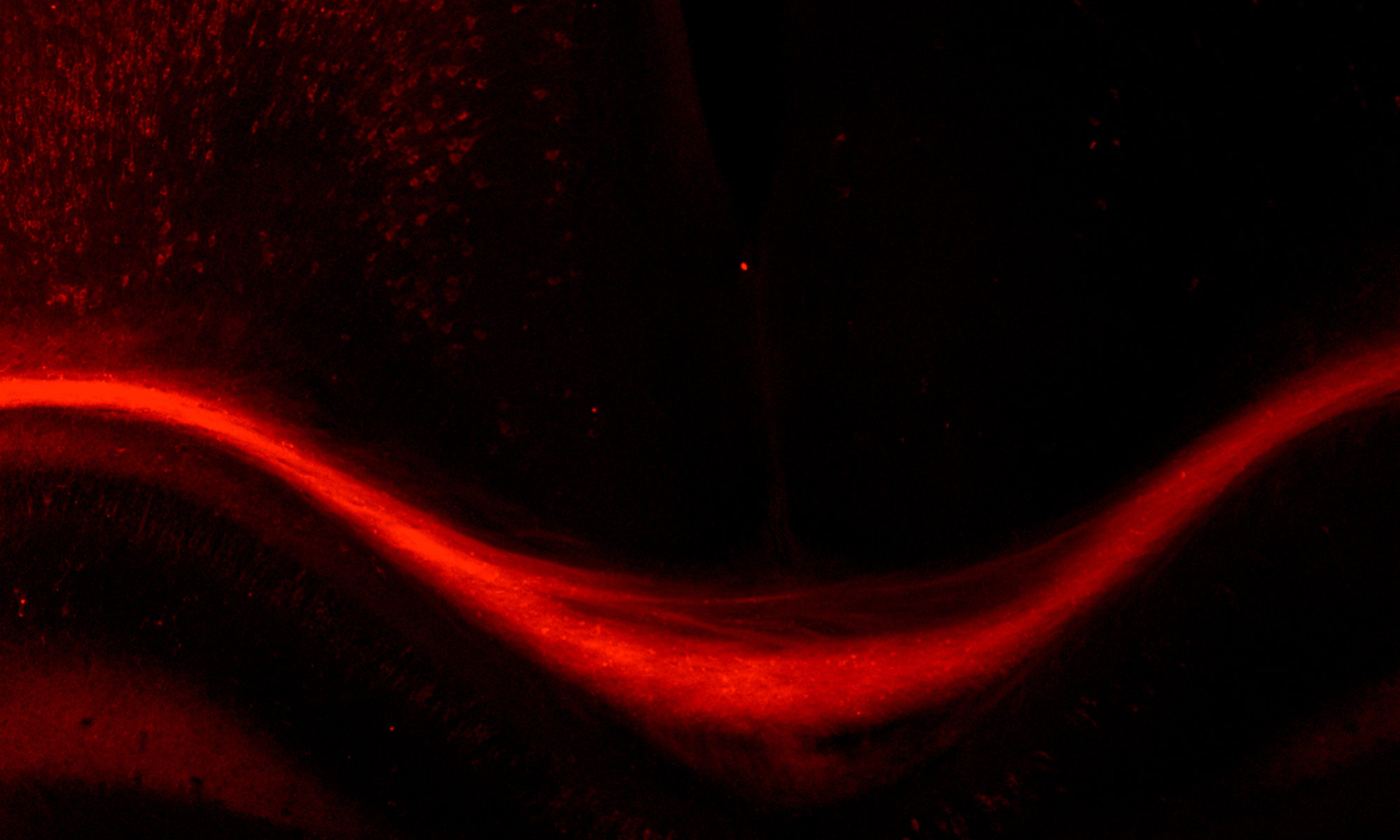
To tease apart how defined environmental factors intersect with these epigenetic factors to modify severity of neurological disorders, we focus on the mammalian neocortex, the area of the brain responsible for much of the high-level cognitive processing. In particular, we study the neurons that connect the two hemispheres of the cerebral cortex – the callosal projection neurons.
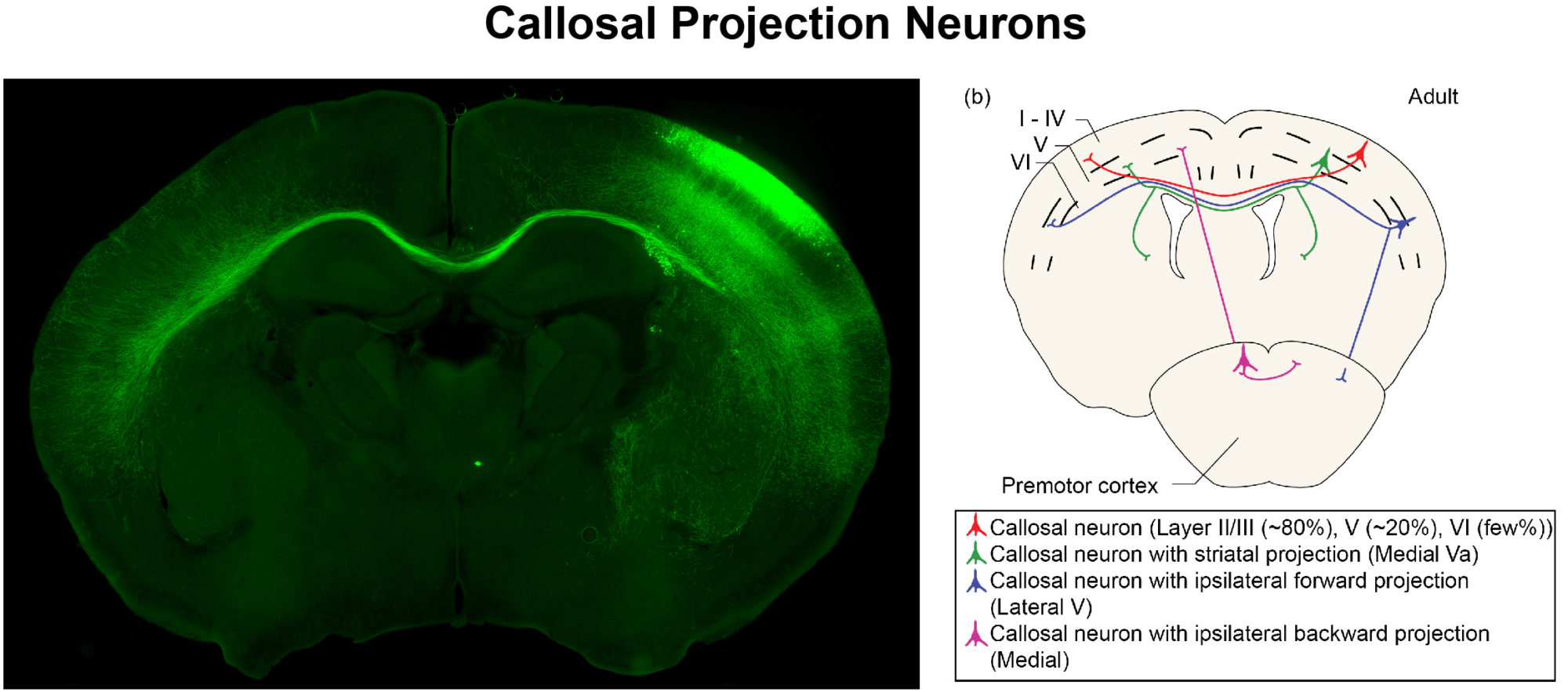
We examine these intersections at multiple stages of development, from embryonic progenitors through to axonal and dendritic connectivity of mature neurons. We use a broad range of experimental approaches, including transcriptome (RNA-seq) and epigenome (Methyl-Seq) analyses on purified neuron and progenitor subpopulations, in vitro models, molecular and biochemical approaches, immunohistochemistry, analysis of neuronal morphology with Golgi staining, axonal tracing, and behavioral analyses.

Interested in joining us?
We have a limited number of openings for exceptional postdocs, graduate students, and undergraduate researchers. Contact Dr. MacDonald directly (jemacdon@syr.edu) if you are interested.